
The Use and Safety of Umbilical Cord Tissue In Inflammatory Diseases, Wellness and Hair Regeneration
The goal in Regenerative Medicine is to “regenerate not operate”, “repair not replace” and “modify or slow down” the progression of chronic disease by harnessing your body’s natural ability to heal itself.
The common denominator of chronic disease is inflammation. Inflammation causes heart disease, autoimmune disease, cancer and increases the aging process. Reducing inflammation is a key to improving health and slowing down the insidious progression of chronic disease. Over half of the 240 registered (phase 1-3) studies on MSC are focused on the anti-inflammatory properties of auto-immune diseases.
The “main function” of Mesenchymal Stem Cell (MSC) in adult tissue is to repair and regenerate the tissue in which they reside. Stem cell “plasticity” refers to the ability of adult stem cells to acquire mature phenotypes that are different from their tissue of origin.
In 1974, umbilical cord blood was declared to be the source of hematopoietic stem and progenitor cells¹ and the remaining umbilical cord tissue was considered medical waste with no scientific value. This point of view was completely revised in 1991, when McElreavey et al. isolated fibroblast-like cells from Wharton’s jelly and characterized them².
In 2004, these fibroblast-like cells were proven to be MSCs as they expressed CD29, CD44, CD51, CD73, and CD105, lacked expression of CD34 and CD45, and had the ability to differentiate into cells of the adipogenic and osteogenic lineages ³.
Currently, the FDA has registered dozens of clinical trials (phases 1–3) on the safety and efficacy of allogeneic unmodified UC-MSCs transplantation for the treatment of socially significant diseases including acute myocardial infarction, cardiomyopathies, critical limb ischemia, bronchopulmonary dysplasia in infants, HIV infection, diabetes mellitus types I and II, both acute and chronic liver diseases, autoimmune hepatitis, cirrhosis of various etiologies, ulcerative colitis, severe aplastic anemia, Alzheimer’s disease, systemic lupus erythematosus, rheumatoid arthritis, myelodysplastic syndrome, hereditary ataxia, spinal cord injury, ankylosing spondylitis, osteoarthritis, multiple sclerosis, Duchenne muscular dystrophy, acute and resistant to steroid therapy for “graft versus host” reactions, and other diseases.
MSC’s injected intravenously do not implant or become end-organ tissue, but lodge in the lung where they are activated to secrete anti-inflammatory protein (TSG-6) which is likely the anti-inflammatory factor that induces the beneficial effects in auto-immune inflammatory diseases.
The purpose of Melhing’s 2015 study was primarily to monitor the immune response to validate the safety of intravenous use and secondly, to evaluate effects on biomarkers associated with chronic inflammation. ⁴ of the more than 200 registered clinical trials for evaluating MSC therapy, 22 are on autoimmune diseases. ⁴
If the infusion of stem cells with 50% probability can lead to changes of blood markers (50% is the maximum entropy), with 99.2% of probability it is reasonable to conclude that this treatment doesn’t lead to essential changes in blood markers and the stem cell treatment was safe for the patients⁴.
Follow up protocols from 9 patients showed increase in energy level (from 33,3±16,7% at 24 hours to 66,7%±16,7% at 3 months), hair, skin improvement and nail grow (from 11,1%±11,1% at 2 weeks to 44,4%±17,6% at 3 months). ⁴ in summary, intravenously administered human umbilical cord stem cells were safe and effective in the treatment of symptoms related to chronic inflammation. ⁴
Moreover, other studies have concluded that the use of umbilical cord is a source of MSCs that have no ethical problems with obtaining biomaterial, they have significant proliferative and differentiation potential, lack of tumorigenicity and have a high immunomodulatory activity.
Umbilical cord MSCs (UC-MSCs) for stem cell therapy has advantages over bone marrow MSCs (BM- MSCs) because they are easily available, collection from the donor is not invasive or painful, and there are no ethical considerations. In addition, UC-MSCs are a higher source of MSC than blood, bone marrow and adipose tissue. ⁵ (Figure 1)
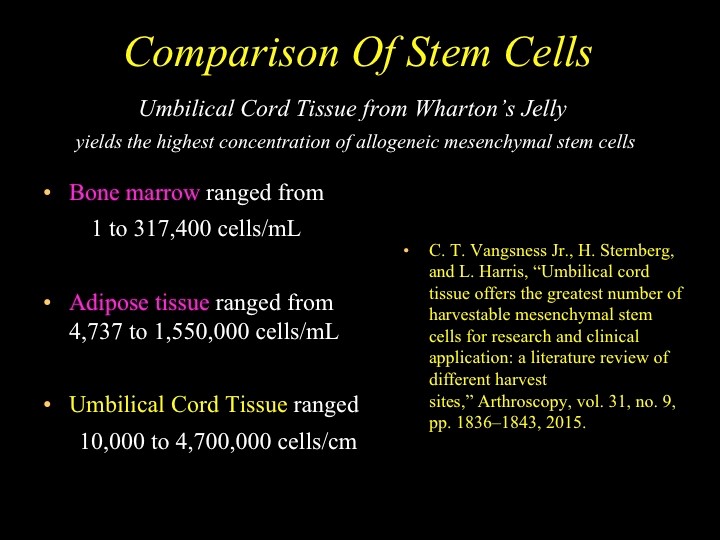
Figure 1
Inflammation also causes hair loss in numerous autoimmune diseases like Alopecia Areata (AA), Discoid Lupus (DL) and Lichen Planopilaris (LPP) and is an important factor in male and female Androgenic Alopecia. Reducing systemic inflammation and reversing its harmful effects on end organ destruction is a key component in modifying disease and improving the symptoms of disease and general wellness.
Fukuoka et al demonstrated that hair regenerative therapy with adipose derived stem cell infusion “was effective for hair growth and is a potential alternative for hair regeneration in patients who are unwilling or unsuitable to undergo traditional surgical hair transplantation”. ⁶
“Our hair follicles are constantly recycling: when a hair falls out, a portion of the hair follicle has to grow back,” said Michael Rosenblum, MD, PhD, an assistant professor of dermatology at UCSF and senior author on the new paper. “This has been thought to be an entirely stem cell-dependent process, but it turns out Tregs are essential. If you knock out this one immune cell type, hair just doesn’t grow.”⁷ Rregulatory T Cells (Tregs) a subpopulation of T cells which modulate the immune system, maintain tolerance to self-antigens, and prevent autoimmune disease)
The new study, published online May 26, 2017 in Cell, suggests that “defects in Tregs could be responsible for alopecia areata, a common autoimmune disorder that causes hair loss and could potentially play a role in other forms of baldness, including male pattern baldness”. ⁷
“We think of immune cells as coming into a tissue to fight infection, while stem cells are there to regenerate the tissue after it’s damaged,” M. Rosenbloom said, “But what we found here is that stem cells and immune cells have to work together to make regeneration possible”. ⁷
“Moreover, MSCs have also been shown to produce anti-inflammatory molecules which can modulate humoral and cellular immune responses. Considering their regenerative potential and immunoregulatory effect, MSC therapy is a promising tool in the treatment of degenerative, inflammatory, and autoimmune diseases”. The reported side effects of increased hair growth, skin improvement, increased nail growth and wellness are all promising and warrant further review.
Patient, Daniel G., Post IV MSC/ CRP and Scalp CRP one month
Scalp / Hair – hairs are growing in frontal region outside / inside hair line and are more visible. Other growth is observed where new hairs are growing out of existing follicles (2 hairs per follicle). Hair loss still minimal and hair is beginning to show signs of thickening.
Neurological – Mental acuity continues to improve (attention, retention comprehension), Deeper sleep, feel rested when I wake up. I’ve had 5 minor concussions in 40 years. It feels like the potential damage associated with concussions are being reversed.
Muscular / Skeletal – Muscle strength and density continues to improve.
GI – Less GI dysfunction.
Stamina / Energy – Libido / performance is improving.
Reference
Knudtzon S. In vitro growth of granulocytic colonies from circulating cells in human cord blood. Blood. 1974;43(3):357–361. Bongso A., Fong C.-Y. Phenotype and differentiation potential of stromal populations obtained from various zones of human umbilical cord: an overview. Stem Cell Reviews and Reports. 2013;9(2):226–240. doi: 10.1007/s12015-012-9418-z. Knudtzon S. In vitro growth of granulocytic colonies from circulating cells in human cord blood. Blood. 1974;43(3):357–361. Brian M. Mehling, Louis Quartararo, Marine Manvelyan, Paul Wang, Dong-Cheng Wu Safety Study of Intravenously Administered Human Cord Blood Stem Cells in the Treatment of Symptoms Related to Chronic Inflammation. World Academy of Science, Engineering and Technology International Journal of Medical, Health, Biomedical, Bioengineering and Pharmaceutical Engineering Vol:9, No:7, 2015 T. Vangsness Jr., H. Sternberg, and L. Harris, “Umbilical cord tissue offers the greatest number of harvestable mesenchymal stem cells for research and clinical Fukuoka, H. Suga, K. Narita, R. Watanabe, S. Shintani. The Latest Advance in Hair Regeneration Therapy Using Proteins Secreted by Adipose-Derived Stem Cells. The American Journal of Cosmetic Surgery 2012; 29:4. Rosenblum, MD, PhD, 2017. On-line pub UCSF News CenterNew Hair Growth Mechanism Discovered, Faulty Immune H. Cells May Play Role in Alopecia, Other Forms of Baldness, published on-line May 26 in Cell
The best way to evaluate a patient is to arrange a personal, in-depth consultation. However, this is impossible for many patients who fly in from other parts of the country or world. For this reason we have developed the remote consultation package, which is an acceptable alternative to the in-person personal consult.
Once your photos and questionnaire are reviewed, we will telephone you to discuss your questions or concerns regarding the procedure. You will also be given a suggested treatment plan and the costs involved. Click here for more complete information on our free on-line hair restoration consultation process.
