23
Feb
Artificial Intelligence based Hair Restoration Studies
Advanced technology in all phases of hair restoration is the gold standard at Greco Hair Restoration.TrichoLAB is an exclusive laboratory specializing in hair research and methods for evaluating hair and scalp disorder so we utilize the Tricholab LAB to objectively assess hair i...
View More26
Jan

Hairline Artistic Design
The most important questions any patient should ask when consulting a hair transplant surgeon for permanent hair restoration are, “What is your goal with hairline restoration” and “What are the Artistic Design principles you utilize to achieve that goal”?...
View More11
Jan
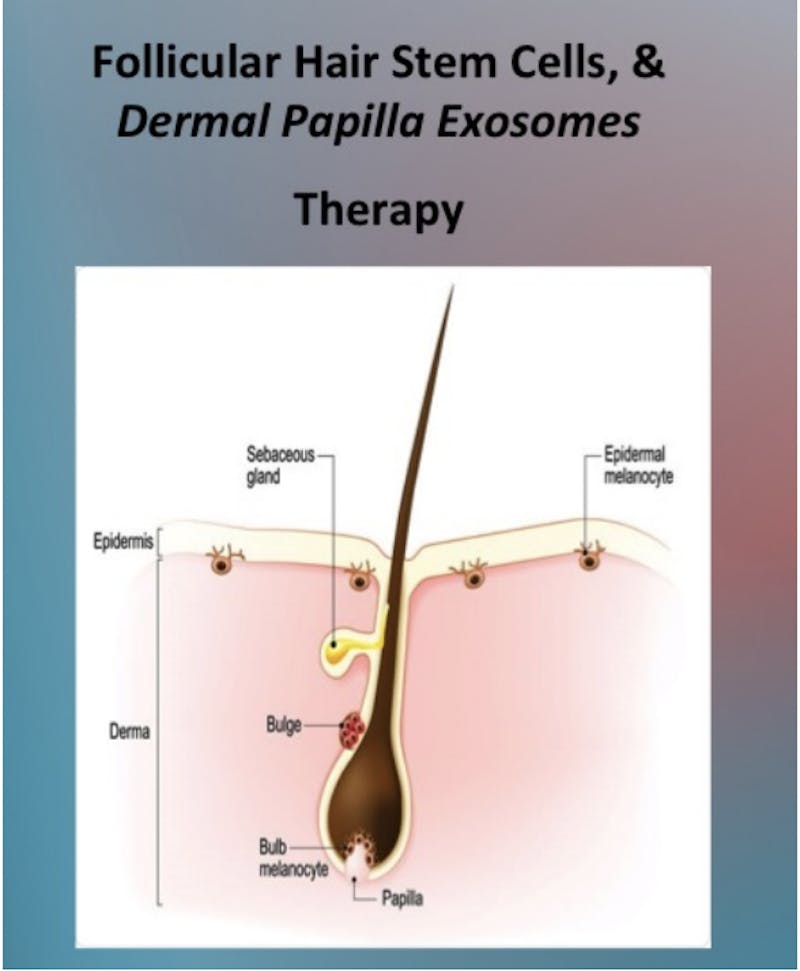
What are Dermal Papilla Exosomes?
Dermal papilla exosomes are tiny packages of proteins, RNA, and other molecules released by the cells in the dermal papilla, a structure located at the base of hair follicles. These exosomes have been found to play a crucial role in promoting hair growth...
View More16
Nov

Regenerative Therapy for Sports Injuries: Fast-Tracking Recovery
In the world of sports, injuries are an unavoidable reality. From muscle strains to joint pain, athletes often find themselves on the sidelines, eager to get back into action. Traditional approaches to injury recovery involve rest, ice, compression, and elevation (R.I.C.E.). Howe...
View More12
Oct

Hair Loss in Menopause: Hormonal Changes and Hair Restoration Options
Menopause is a natural phase in a woman's life that is characterized by significant hormonal changes. While patients are aware of most of the various physical and emotional transformations that come with it, one aspect that often goes unnoticed is hair loss. Understanding w...
View More04
Oct

Our OATH: Oxygen-Amplified Therapy
OATH OXYGEN AMPLIFIED THERAPY is an oxygen transportation method that can carry 20 times more molecular oxygen than water, releasing deep into the skin's cells, resulting in firmer and more radiant skin.As skin ages, the skin cells lose oxygen, a leading cause of fine lines, wri...
View More18
Sep

Choosing the Right Hair Transplant Surgeon: Expertise Matters Most
18 September: Choosing the Right Hair Transplant Surgeon: Expertise Matters MostHair loss can be a challenging experience, impacting your appearance, self-esteem, and confidence. If you've decided that a hair transplant is the right solution, congratulations on taking that step...
View More09
Aug

Empowering Recovery: Advanced IV Therapy for Athletes
9 August: Empowering Recovery: Advanced IV Therapy for AthletesIn the world of athletics, optimal performance and quick recovery are essential for success. Athletes are always pushing their bodies to the limit, subjecting them to physical stress, dehydration, and nutrient deplet...
View More11
Jul

Comparing CRP Therapy with Traditional Pain Management
11 July: Comparing CRP Therapy with Traditional Pain ManagementPatients who live with chronic pain find that it has a major impact on their quality of life. Traditional pain management approaches, such as medication and physical therapy, have long been the go-to methods for alle...
View More06
Jul

Microblading vs. Eyebrow Transplant: Which Is Better?
23 May Microblading vs. Eyebrow Transplant: Which Is Better?Posted at 12:46 PMin Blog by Joe Greco If you want to enhance your eyebrows' appearance, microblading, and an eyebrow transplant are great options, but which is better?Both microblading and eyebrow transplants...
View More